With any cancer diagnosis, the one factor that determines the entire path forward is whether it was detected early enough. This is especially poignant with lung cancer. Lung cancer is the second most common cancer in both men and women in the US, and is the leading cause of cancer-related deaths worldwide. [1] Early detection is a first sign of hope that the disease will be “easier” to treat, more responsive to treatments, and result in a higher survival rate. So at what point is a cancer detected early enough?
Understanding Cancer Staging
The AJCC (American Joint Committee on Cancer) developed a cancer cell classification system called the TNM Staging System, which helps an oncology team evaluate a cancer prognosis (outcome) and design an appropriate treatment plan with the hope of achieving a progression-free survival (PFS).
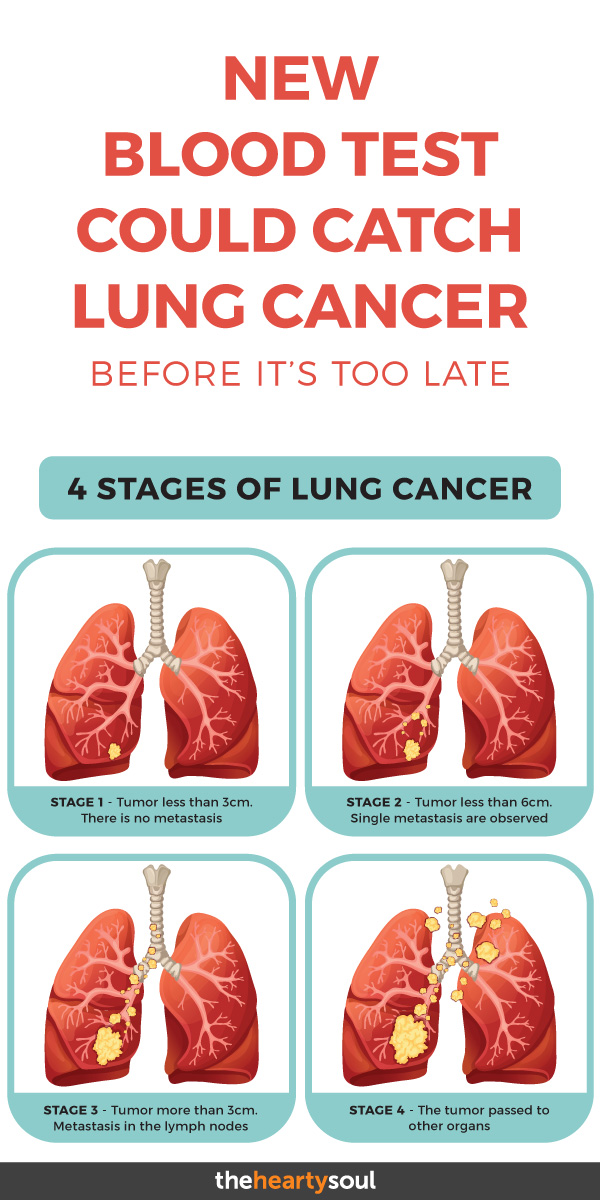
The TNM system is used to determine the extent of a tumor (T) as well as whether or not cancer has spread to any lymph nodes (N) or spread beyond its original site and invaded other parts of the body in the form of metastases (M). Once a TNM class is determined, an overall Stage 0, I, II, II or V is assigned, and these may be subdivided further into, for example IIA and IIB, where the earlier number is a lower stage.
Stage 0, also known as carcinoma in situ, is very early detection, while Stage IV means that the cancer is advanced and has spread to other body parts or organ systems. [2] Each cancer type however may have its own unique system, or letter and number combinations.
Based on its histology, lung cancer is categorized either as lung carcinoid tumor (about 5%), small-cell lung cancer (10-15%) or non-small cell lung cancer (NSCLC, about 85%). [3] About 57% of NSCLC are already at stage IV at initial diagnosis. [1] Here are some examples of estimated 5 year survival rates based on the staging for (NSCLC). [4]

Early detection is thus imperative to improve the chances of successful, curative treatment and a progression-free survival rate for a vast majority of NSCLC diagnoses.
Traditional Lung Cancer Screening Methods
How do we secure our chances of an early detection? The symptoms of lung cancer can often be mistaken for a lingering infection (mainly in non-smokers) or the associated after effects of years of smoking. Once we notice symptoms, these can often be indicative of a more advanced stage already. Sometimes a diagnosis is stumbled upon because we are being tested for some other condition entirely.
Standard screening methods traditionally use imaging technology or tissue biopsies. According to the American Cancer Society, research has shown that using chest low-dose CAT (LDCT) scans to screen people at higher risk of lung cancer saved more lives compared to chest x-rays.
The National Lung Screening Trial (NLST) of 2002-2004 demonstrated a 20% mortality rate reduction in patients who had undergone LDCT screening, compared to patients screened with a conventional chest X-ray [5] For higher risk people, getting yearly LDCT scans before symptoms start helps lower the risk of dying from lung cancer.
However, even though (LDCT) is recommended by the U.S. Preventive Services Task Force (USPSTF) for people with significant smoking history, it is vastly underutilized. [6]. Further, screening with LDCT will not find all lung cancers, and not all of the cancers that are found will be found early.[9]
In addition, it was found that LDCT had a high false-positive rate, resulted in unnecessary psychological anxiety and was associated with the development of radiation-induced lung cancer due to repeated radiation exposure.[5]
Other standard lung cancer screening involves a sputum cytology which is a test where a coughed-up sample of mucus is analyzed to see if it contains cancer cells. A more invasive approach is a biopsy which involves collecting a tissue sample in one of three ways: either by passing a tube down the throat into the lung, by inserting a needle through the chest wall or by minor surgery. [9,10]
These methods of screening are, generally, only applied when there already exists a suspicion of a possible lung cancer diagnosis. A tissue sample, however, is an important tool in pathology to determine staging and treatment options. Nevertheless, a biopsy can be uncomfortable to the patient who often will require local anesthesia and may be difficult to perform due to the location of a suspected tumor.
A New Approach to Testing using Molecular Diagnostics
In 2016 the Food and Drug Administration (FDA) approved a new lung cancer screening test method that uses genomic sequencing. It involves a simple blood draw with subsequent analysis of blood plasma – a liquid biopsy. Cancer cell-related information can be extracted from blood plasma allowing for a less invasive, simpler procedure.
A variety of circulating cancer biomarkers and genetic material can be isolated and subsequently analyzed, including mutated circulating tumor DNA which are tiny cell-free DNA fragments (cfDNA) shed by the tumor. [8] Others include tumor-associated antigens (TAAs), tumor-associated autoantibodies (TAAbs), circulating tumor cells (CTCs), microRNA (miRNA) and exosomes. [5]
A liquid biopsy was originally approved to help identify patients with metastatic NSCLC who would qualify for a targeted treatment, erlotinib (Tarceva). [11] This test is also useful to monitor treatment response and disease progression, as the nature of cancer tumors can change while undergoing treatment, and it is imperative to catch this early in order to adapt a treatment plan.
Initially, liquid biopsy technology was limited to serving as an adjunct to existing traditional screening methods, such as CT scans. However, molecular technology is advancing at such a rapid rate, that the focus has now shifted to replacing CT scans as a new lung cancer screening approach. The ease with which a liquid biopsy can be performed and repeated, coupled with advancing technologies that can analyze even small amounts of biomarkers for possible genetic mutations even before the presence of abnormal images, makes this a feasible test to also apply as a screening tool for early detection of lung cancers. This would also greatly reduce the current rate of overdiagnosis and radiation risk associated with CT scans. [5]
The role that molecular pathology and genome sequencing will play in the management of cancer as a whole, and hopefully reduction in cancer cell deaths through early detection, is steadily becoming more apparent.
A large scale global initiative by GRAIL, Inc. has attracted global funding of over $1.5 billion since 2016 to develop early cancer detection methods for a variety of cancers utilizing genomic molecular testing technology such as liquid biopsy.
The goal is to develop technology that can detect cancer cells before a tumor has formed or symptoms are noticeable. Grail’s CEO, Jennifer Cook, said in a statement that the firm recently reported data supporting the potential for development of a highly specific and sensitive blood test, to optimize and validate a product for early detection of multiple cancer types. [7, 14]
GRAIL, Inc. is funding a large, ongoing Circulating Cell-Free Genome Atlas (CCGA) study that is providing preliminary evidence that genome sequencing via blood testing may be used to detect early-stage lung cancer. Until recently, liquid biopsies have been used only for people with advanced lung cancer, and the evidence has been limited to show that this method may be feasible for early detection of lung cancer. Additional larger data studies were needed with people who have not been diagnosed with cancer.
At the June 2018 annual meeting of ASCO (American Society of Clinical Oncology), CCGA lead study author Geoffrey R. Oxnard, MD, Associate Professor of Medicine at Dana-Farber Cancer Institute and Harvard Medical School in Boston, MA. had this so say:
“We’re excited that initial results from the CCGA study show it is possible to detect early-stage lung cancer from blood samples using genome sequencing. There is an unmet need globally for early detection tests for lung cancer that can be easily implemented by health care systems.” [6, 7, 8]
Other research is looking at the usefulness of using liquid biopsy tests to help determine possible recurrence and disease progression before standard screening methods such as CT scans would confirm this. In 2017 a study in the UK involving 100 patients with non-small cell lung cancer were followed from diagnosis through surgery and chemotherapy.
Head of the study, Prof. Charlie Swanton, a cancer geneticist at the Francis Crick Institute, confirmed how analyzing circulating tumor DNA could track a patient’s disease status with remarkable precision. Of patients who would remain in remission, he said that within 48 hours of surgery, the DNA dropped down to undetectable levels in those patients who remained in remission.
By contrast, rising tumor DNA levels were seen in patients whose disease would later recur, indicating that cancer remained in the lungs or had migrated to other organs, where it was lying dormant. The scientists could say with 92% accuracy who would relapse. [17]
Researchers at Johns Hopkins University Kimmel Cancer Center developed a liquid biopsy single blood test called CancerSEEK that is able to simultaneously detect levels of eight cancer proteins as well as the presence of cancer gene mutations from circulating DNA in the blood.
It is hoped that the test could be used in the future in the screening for, and specific location of the possible presence of eight common cancer types that account for more than 60 percent of cancer deaths in the U.S: lung, liver, ovarian, pancreas, stomach, esophagus, colorectum and breast.
The findings were published in January 2018 [15], and according to Nickolas Papadopoulos, Ph.D., senior author and professor of oncology and pathology, “the use of a combination of selected biomarkers for early detection has the potential to change the way we screen for cancer, and it is based on the same rationale for using combinations of drugs to treat cancers.”
The test did have a few false positives in certain of the cancer types, meaning it showed the presence of cancer when there really wasn’t. The study also mainly used people that had already been diagnosed with cancer. [13,15]
It is encouraging to see these promising results that could secure a future in which early cancer detection, as well as treatment efficacy and progression can be monitored with a non-invasive and easy to administer blood test. Before this technology can become accurate and mainstream, further research is warranted.
As Len Lichtenfeld, MD, deputy chief medical officer at the American Cancer Society puts it, “a meaningful liquid biopsy of the future will need to find cancer in someone with no signs of it and be able to determine where that cancer may be in the body. It also needs to tell us whether that cancer requires treatment. One of the keys in this discovery process is not only to find the cancer cell or find the mutated DNA fragment circulating in the blood, but also to find out whether that particular person’s cancer is going to be a problem. We definitely have come a long way, but we still have a long way to go. We don’t know yet whether screenings with a liquid biopsy could help doctors learn how aggressive a cancer is.” [16]
Risk factors and Causes of Lung Cancer?
Smoking is a well known cause and thus risk factor for lung cancer, however the rates of lung cancer diagnoses are high even in people who have never smoked. The Mayo Clinic lists the following risk factors, which can be viewed as underlying causes [18]
- Smoking – the number of cigarettes as well as daily frequency and long-term duration all have an impact.
- Exposure to secondhand smoke
- Exposure to radon gas. Radon is produced by the natural breakdown of uranium in soil, rock and water that eventually becomes part of the air you breathe. Unsafe levels of radon can accumulate in any building, including homes.
- Exposure to asbestos and other carcinogens such as arsenic, chromium, nickel, most often in the workplace.
- Family history of lung cancer. People with a parent, sibling or child with lung cancer have an increased risk of the disease.
What are potential symptoms of Lung Cancer?
Signs and symptoms of lung cancer can vary, here are the most common ones: [18]
- A new cough that lingers for weeks
- Coughing up blood, even a small amount
- Shortness of breath
- Chest pain
- Hoarseness
- Losing weight without trying
- Bone pain
- Chronic Headaches
Having a liquid biopsy blood test done through a simple blood draw at the doctor’s office may improve lung cancer screening rates, but before such a test can be widely used for early detection, additional validation in larger data sets and in studies with people who have not been diagnosed with cancer are needed.
In the meantime, I cannot stress the importance of undergoing a mindset shift. When it comes to early detection, it is simply not enough to rely solely on advancing technology in cancer diagnostics to help us monitor what is going on in our bodies.
We are each responsible for ensuring that we lay a solid foundation with our nutritional and lifestyle choices to strengthen our bodies from within. Here are a few tips I address in my own practice:
- Learn to select, prepare and eat nutrient-dense meals that truly nourish our bodies. Ingredients and cooking techniques matter as these can directly lead to inflammatory processes and an accumulated toxic burden that can set the stage for future cancer growth. We also need to become aware of any nutritional deficiencies and digestive issues if we are to ensure a healthy immune system that can do its job.
- The mind is our most powerful tool when it comes to utilizing successful stress management techniques. Stress is a fact of life, yet we have unlearnt effective techniques to know which stress is life-threatening, and which can be managed and regulated through our own choices.
- We need to get moving! Our bodies are not meant to be sedentary to the degree they are today. Our lymphatic system is a major organ of detoxification, and without adequate muscular contractions it becomes sluggish and unable to function effectively. A little goes a long way, as long as our exercise is consistent. Whether it is stretching, weight-bearing, cardio exercise or muscle toning, get moving!
- Emotional Self-Care is top priority. Underlying every cancer diagnosis is at least one suppressed emotional issue we have tried to ignore, whether we realize it or not. We need professional help to address this, and there are numerous options available to us.
- Finally, nothing in our bodies exists in isolation. Everything is related, and each of us is unique in this regard. It is time to connect within and get to know who we really are as physical, energetic, emotional and spiritual beings. No single diagnostic tool or medical treatment can address all the layers that make up our entire body.
Sources
- 1. Siegel, RL, Miller, KD, Jemal,A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
- 2. https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/staging.html
- 3. https://www.cancer.org/cancer/lung-cancer.html
- 4. https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html
- 5. Liang, Wenhua et al. “Liquid Biopsy for Early Stage Lung Cancer.” Journal of Thoracic Disease10.Suppl 7 (2018): S876–S881. PMC. Web. 11 Oct. 2018. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5945687/
- 6. https://www.asco.org/about-asco/press-center/news-releases/blood-test-shows-potential-detection-tool-early-stage-lung
- 7. https://www.timesnownews.com/health/article/lung-cancer-could-a-blood-test-help-detect-early-signs/235705
- 8. https://www.theguardian.com/science/2018/jun/01/how-does-holy-grail-cancer-test-work-and-when-will-it-be-available
- 9. https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/detection.html
- 10. https://www.mayoclinic.org/diseases-conditions/lung-cancer/diagnosis-treatment/drc-20374627
- 11. https://blog.aacr.org/fda-approval-liquid-biopsy-test-lung-cancer/
- 12. Kwapisz, Dorota. “The First Liquid Biopsy Test Approved. Is It a New Era of Mutation Testing for Non-Small Cell Lung Cancer?” Annals of Translational Medicine5.3 (2017): 46. PMC. Web. 12 Oct. 2018. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5326656/
- 13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6080308/
- 14. https://www.genomeweb.com/sequencing/grail-raises-300m-series-c-round
- 15. https://www.hopkinsmedicine.org/news/newsroom/news-releases/single-blood-test-screens-for-eight-cancer-types
- 16. https://www.cancer.org/latest-news/liquid-biopsies-past-present-future.html
- 17. https://www.theguardian.com/society/2017/apr/26/dna-based-test-can-spot-cancer-recurrence-a-year-before-conventional-scans
- 18. https://www.mayoclinic.org/diseases-conditions/lung-cancer/symptoms-causes/syc-20374620