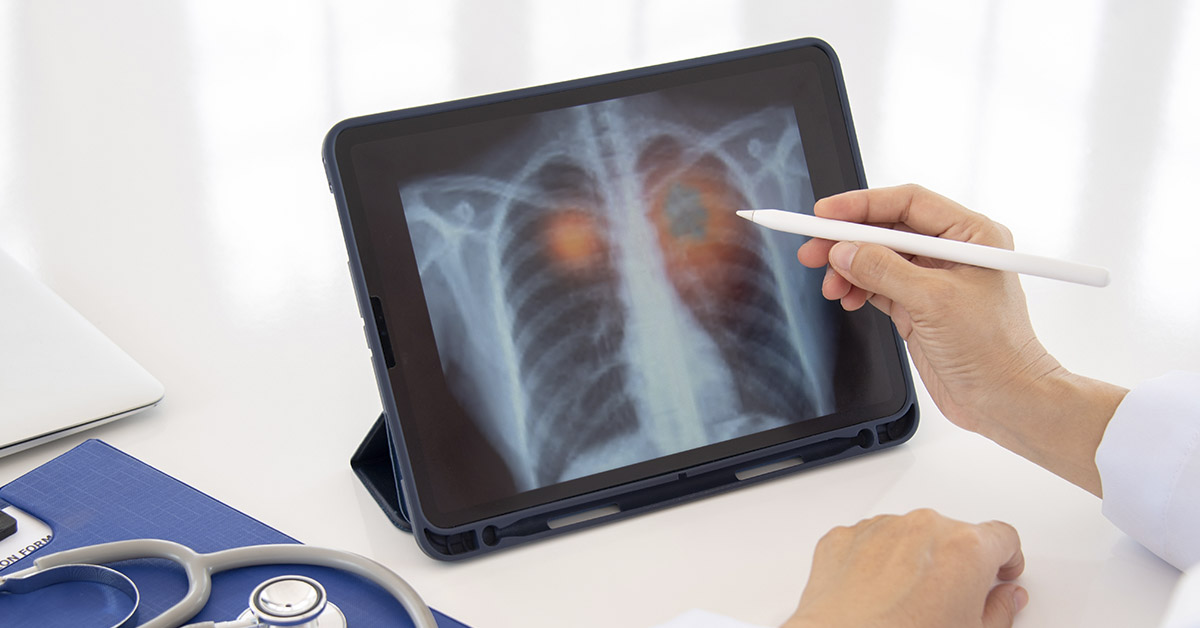
Until very recently, the idea of a lung cancer vaccine would have been dismissed as medical science fiction. However, since August 2024, when the very first lung cancer mRNA vaccine entered human trials, this idea has rapidly become a scientific fact. Additionally, other recent innovations, such as the personalized neoantigen vaccine PGV001, have shown encouraging results. In this article, we will take a closer look at the latest developments and challenges in the promising field of cancer immunotherapy.
The Launch of The First Lung Cancer Vaccine Trial
In August of 2024, BioNTech, a German biotech company, launched the first phase of their trial of an mRNA vaccine that targets non-small cell lung cancer. The trial was launched across seven different countries, including Germany, the United Kingdom, the United States, and Turkey. Around 130 patients had enrolled by early 2025, with the aim of testing safety and immunogenicity alongside immunotherapy. During the interim, reports have suggested that the vaccine, BNT116, has provoked a targeted immune response against tumor antigens. Additionally, the side effects were reported to be tolerable.
mRNA Vaccine Trials Gain Traction
The success of the BNT116 vaccine has inspired the proliferation of similar cancer vaccines. Moderna presented its experimental therapy mRNA-4359 at ESMO 2024. It was shown to elicit immune responses in individuals with lung, melanoma, and other solid malignancies. Even though these trials were small-scale and in their early phases, they confirmed the potential of using mRNA platforms to address many different types of tumors. Thanks to promising results in animal studies and evidence that the immune system is responding well, researchers are now moving toward bigger studies involving more patients.
Read More: Technique Uses Vibrating Molecules to Kill 99% of Cancer Cells in Laboratory Study
The Promising Neoantigen Vaccine
Alongside the cancer vaccines that target the common cancer markers, researchers are also working on the development of personalized vaccines. Researchers at Mount Sinai shared their results on a study on PGV001 – a personalized vaccine that is designed to match the mutations in every patient’s tumor. In early trials involving 12 participants with five different cancer types, they found that the vaccine was safe, with very few side effects. Quite significantly, six of the 12 patients were still alive five years later, and three exhibited no signs of cancer at all.
Vaccine‑Immunotherapy Combinations
To make them work even better, certain cancer vaccines are also often combined with other treatments called checkpoint inhibitors. Mount Sinai researchers are now testing PGV001 alongside a drug known as atezolizumab in patients diagnosed with bladder cancer. Early results have indicated that this particular combination is safe to use and helps activate the immune system. At the same time, companies such as Merck and Moderna are testing their mRNA vaccines in combination with pembrolizumab in individuals diagnosed with lung cancer and melanoma.
Read More: New RSV Vaccine Targets Pregnant Women and Seniors in UK Rollout
The Scalability of Shared Neoantigen Vaccines
Personalized vaccines are extremely precise, but developing them for each individual is challenging and expensive. To address this issue, Mount Sinai has begun testing shared neoantigen vaccinations. Rather than developing a separate vaccination for each patient, these vaccines target mutations that many patients share. This allows them to be produced in larger batches, lowering prices while maintaining a certain degree of customization. The first small trials are currently underway, and this new approach has the potential to reduce the cost and availability of cancer vaccines.
Read More: 12 Groundbreaking Advances in Cancer Research
The Bottom Line on Lung Cancer Vaccines
Strong support networks are driving these scientific discoveries forward. In the United Kingdom, the Cancer Vaccine Launch Pad initiative began at the end of 2022. It accelerates trial approval and increases production, with the objective of treating 10,000 patients by 2030, in collaboration with companies such as Moderna and BioNTech. Many of the methods that were created during the COVID-19 pandemic, such as special cold storage and rapid genetic testing, are used in the program to help minimize the time it takes to begin trials. If early results continue to be promising, several of these cancer vaccines may be approved by late 2025 or early 2026. This kind of government assistance is vital for making these vaccinations more broadly available.
Read More: Mouth Cancer: Symptoms, Signs, and When to See a Doctor